Introduction
Financial toxicity (FT) in cancer care is a growing public health problem faced by patients, caregivers, and oncologists . This burden is more pronounced in hematologic malignancies (HM), given the intense nature and novel but costly therapies in this patient population.Underserved communities face disproportionately higher financial toxicity of cancer treatment. This study aims to determine the impact of sociodemographic factors and clinical characteristics on the severity of FT in patients treated for HM, largely from underserved communities in a tertiary care hospital in the Bronx.
Methods
This prospective ongoing survey-based study is being carried out at the Montefiore Medical Center, Bronx, NY. All patients were adults categorized into newly diagnosed (ND) patients with HM and those receiving cellular therapy (CT): autologous/allogeneic stem cell transplantation and CAR-T cell therapy. Investigator designed questionnaire was used to obtain sociodemographic and clinical characteristics of the patients, while The Comprehensive Score for Financial Toxicity (COST-FACIT) questionnaire, a validated FT tool, was used to determine the severity of FT for each patient. Patients were given a score based on their answers for the COST-FACIT questionnaire: score 0 = Grade 3 - severe FT; 1-13 = Grade 2 - moderate FT; 14-26 = Grade 1 - mild FT and >26 = Grade 0 - no toxicity. Surveys were administered on day 0, day 30, and day 90. We looked at mean FT scores according to sociodemographic factors, disease type, and treatment arms. The student's t-test ANOVA was used for statistical analysis using SPSS software. This study was approved by the institutional IRB.
Results
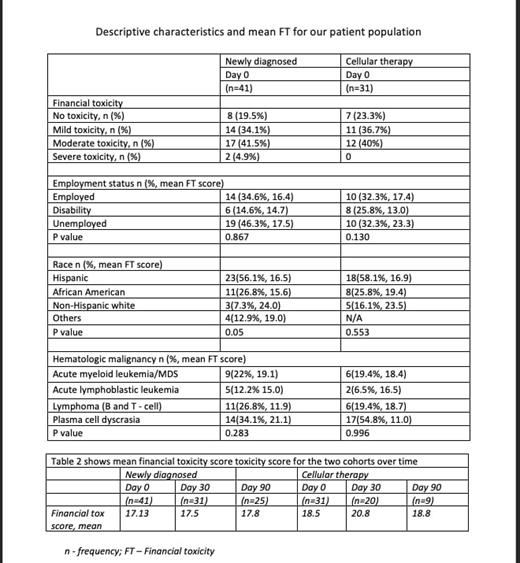
We have recruited a total of 72 patients at the time of analysis to date (Table). At Day 0, there were 41 patients in the ND group and 31 patients in the CT group. In the ND group, 31 and 25 of them had completed the day 30 and day 90 survey respectively, while in the CT group, 20 and 9 patients completed the day 30 and day 90 survey, respectively. Seven patients (4 in ND, and 3 in CT cohort) died at the time of their first survey. The mean FT for various demographic and clinical characteristics is summarized in the table. We obtained further information using our questionnaire and correlated it to the COST-FACIT score. On day 0, 10 patients (24.4%) in the ND cohort and 5patients (16%) in the CT cohort reported having difficulty paying for food, shelter, and clothing often to most of the time. Over 90% of these patients reported moderate to severe FT on the COST-FACIT survey .On day 0, 10 patients (24.4%) of ND patients and 10 patients (32.3%) of CT patients reported delays in seeking medical care or missing medications due to inability to pay. Seventy percent of these patients had moderate to severe FT on day 0. On enquiring about emotional well-being over the last four weeks, 16% (6 in the ND cohort, 6 in the CT cohort) reported feeling depressed, anxious, depressed, and sad often to most of the time. 83% of those had moderate to severe FT. Comparing the mean FT longitudinally between the two arms, most patients experienced mild FT, but the CT group on Day 30, had a higher FT than on Day 0.
Conclusion
Our results show that a sizeable proportion of patients in the Bronx report financial strain at baseline that leads to emotional stress, difficulty in paying for food, basic necessities and leads to delay in seeking medical care or skipping medications. We also see that patients who are referred for CT potentially have worse financial toxicity over time. This group may be even more vulnerable given that CT is offered at select FACT-accredited centers and patients may have to travel longer distances. Our data is capturing only those patients who were able to make it to CT and there may be a selection bias introduced by the nature of this data. We will report further follow-up data at the meeting considering disease-specific treatments and aim to design specific interventions for this patient population.
Disclosures
Mantzaris:Kite, a Gilead company: Honoraria. Konopleva:Abbvie, Allogene Therapeutics, Cellectis, Forty Seven, Gilead Sciences, Genentech, Sanofi, MEI Pharma, Rafael Pharmaceuticals, Daiichi Sankyo Pharmaceutical, AstraZeneca Co., Menarini, Precision BioSciences.: Research Funding; Reata Pharmaceuticals.: Current holder of stock options in a privately-held company, Patents & Royalties; AbbVie, Forty Seven, Precision Biosciences, Gilead Sciences, Genentech, Janssen, Sanofi, MEI Pharma, Daiichi Sankyo Pharmaceutical, AstraZeneca Co., Menarini.: Consultancy. Verma:Eli Lilly: Research Funding; Stelexis: Current equity holder in private company, Honoraria, Other: Scientific Advisor; GSK: Research Funding; Incyte: Research Funding; Medpacto: Research Funding; Curis: Research Funding; Bakx: Current equity holder in private company, Other: Scientific Advisor; Novartis: Other: Scientific Advisor; Acceleron: Other: Scientific Advisor; Celgene: Other: Scientific Advisor; BMS: Research Funding; Prelude: Research Funding; Janssen: Honoraria; Throws Exception: Current equity holder in private company. Shastri:Gilead Sciences: Honoraria; Rigel Pharmaceuticals: Honoraria; Kymera Therapeutics: Honoraria, Research Funding; Janssen Pharmaceuticals, Inc.: Consultancy, Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal